
Lewis Structure For C2h6 Drawing Easy
The correct Lewis structure for ethene is shown below: For more information on how to use Lewis Dot Structures refer to http://chemwiki.ucdavis.edu/Wikitext.wis_Structures.
(Get Answer) Wu.Edu/ A Ball And Stick Model Of Ammonia (NH3) Is Shown
Molecular formula. The molecular formula tells the symbols of the elements that compose the compound, and the subscript to the element symbol denotes how many atoms of that element are in the molecule.. For example, (\(\ce{CH4}\)) is a molecule formula of methane which means there is one carbon and four hydrogen atoms in a methane molecule. \(\ce{C2H6}\) is a molecule formula of ethane, which.
C2h6 Lewis Structure Shape Draw Easy
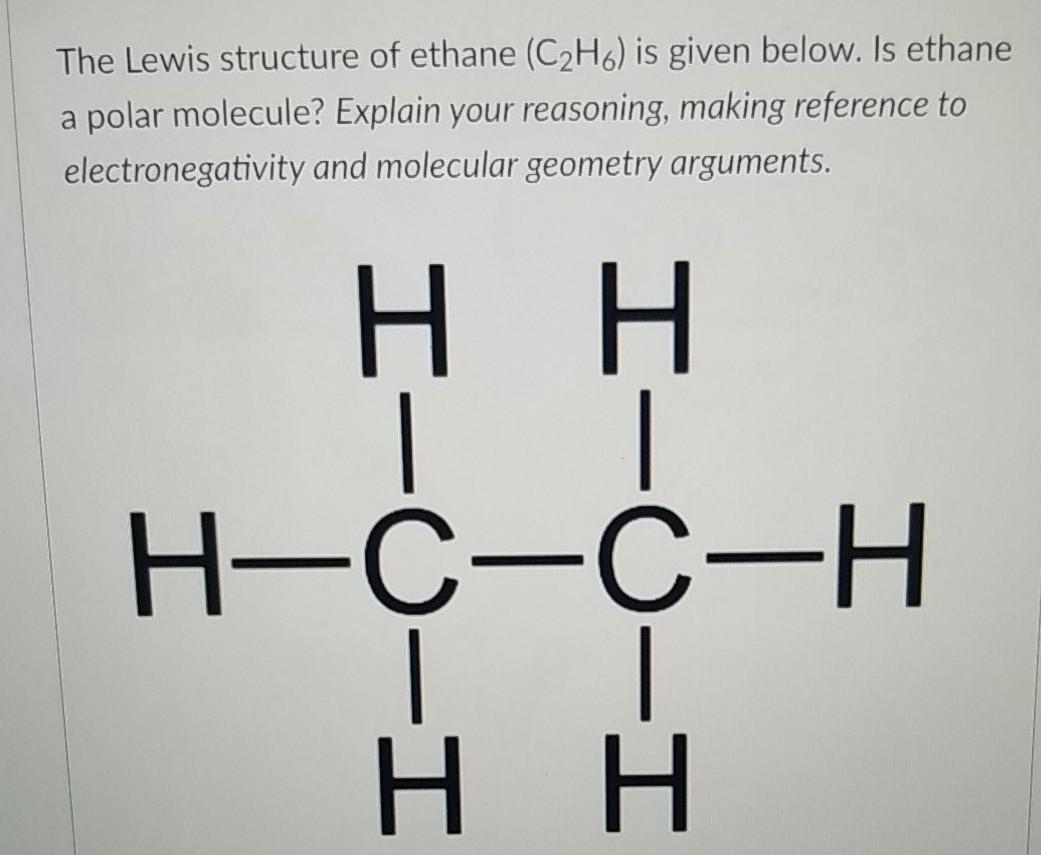
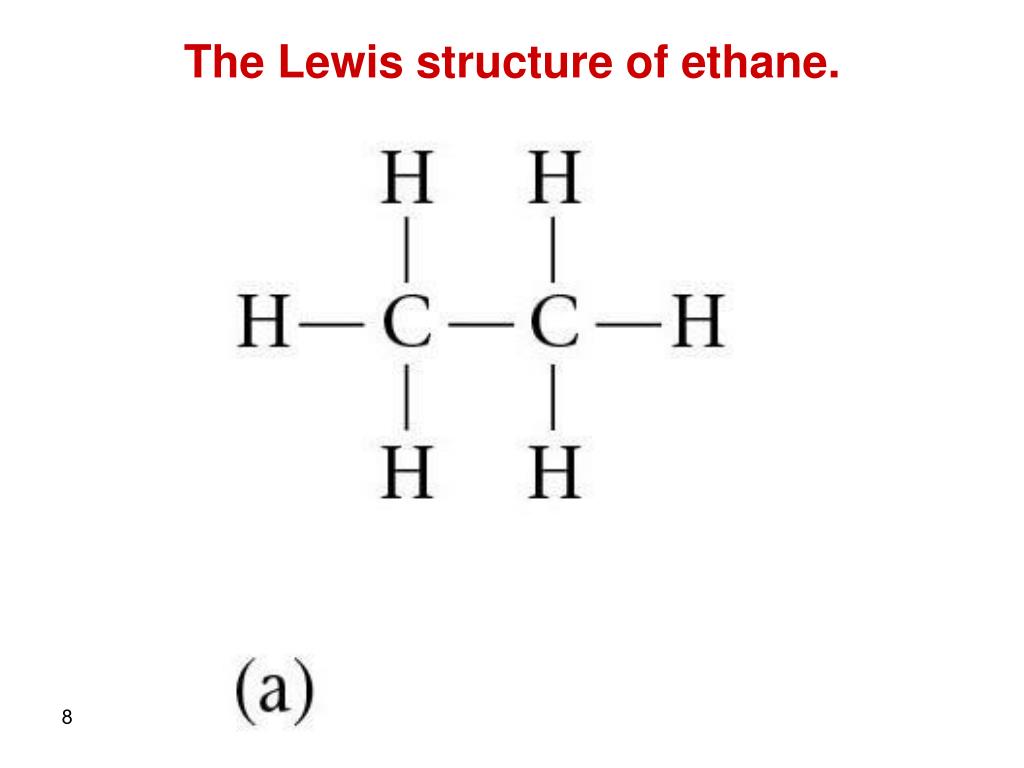
Lewis structure of C2H6 (or Ethane) contains single bonds between Carbon-Carbon atoms as well as between Carbon-Hydrogen atoms. The two Carbon atoms (C) are at the center and they are surrounded by 3 Hydrogen atoms (H). Let's draw and understand this lewis dot structure step by step.

Lewis Structure Of Ethane
A quick explanation of the molecular geometry of C2H6 including a description of the C2H6 bond angles.We'll determine the C2H6 molecular geometry with respec.
14+ C2H6 Lewis Structure Robhosking Diagram
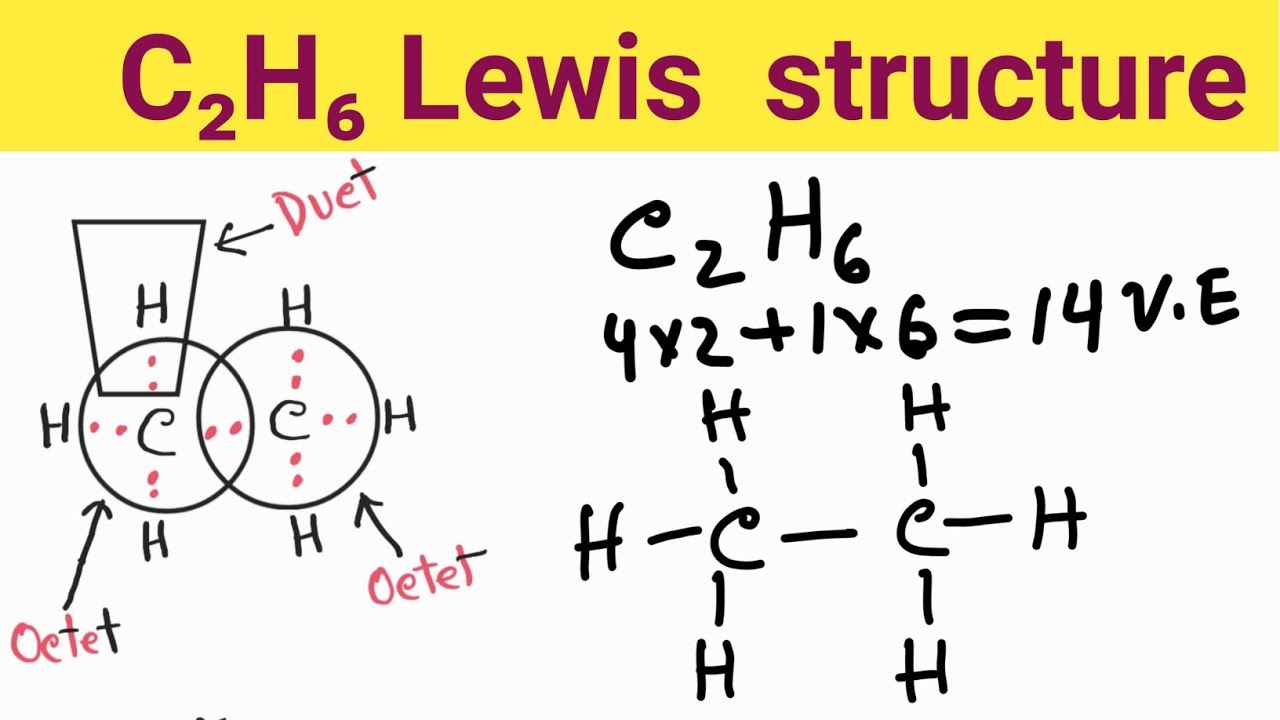
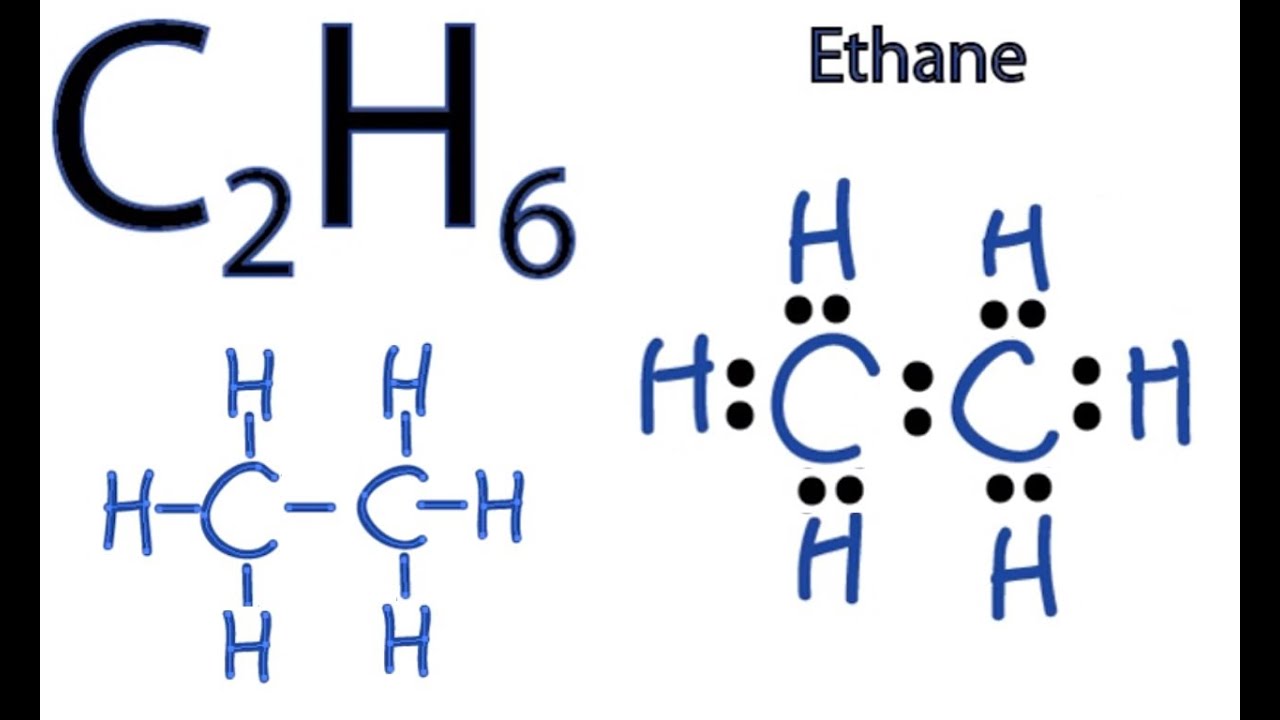
The Lewis structure of ethane would be: We can count the total number of valance electrons in the Lewis structure of ethane, which is equal to 14. The total number of electrons around each carbon atom is 8 electrons and hence, it has completed its octet. Every hydrogen atom is surrounded by two electrons, leading to duplet formation.

How to Draw the Lewis Dot Structure for C2H6 Ethane YouTube
Let us draw the Lewis dot structure of Ethane, C2H6: Ethane Lewis dot structure The above picture is the representation of the position of valance electrons in C2H6. This is the basic Lewis dot structure of the molecule. The dots are the symbol of valance electrons. There are two carbon atom participates in this sharing.
C2H6 / IGCSE Chemistry Covalent Substances (Section 1g) Computed by
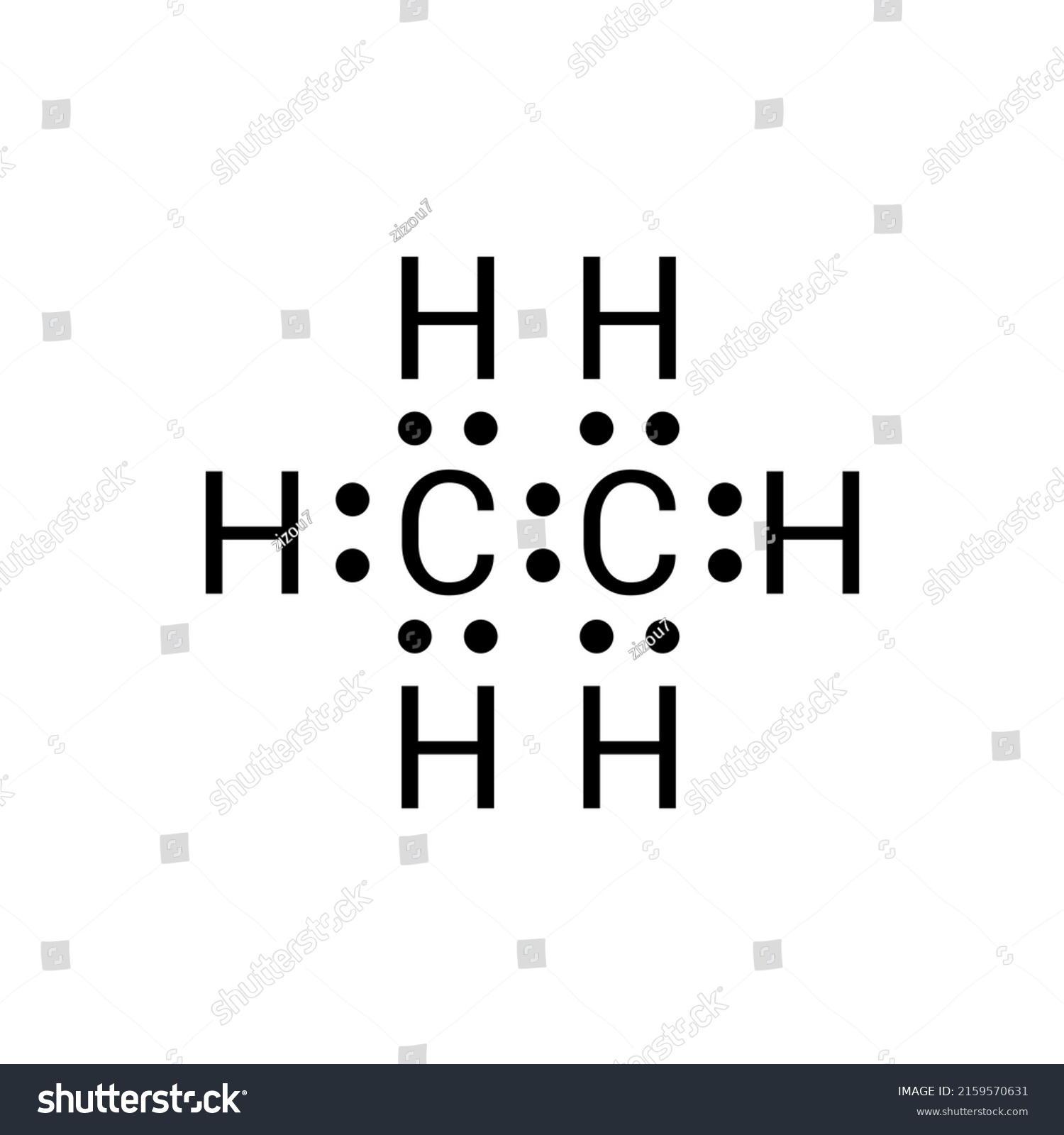
In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. Contents Steps #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms External links Steps
C2H6 Lewis StructureEthane Lewis StructureLewis Dot Structure for
The first step in drawing the Lewis dot structure for ethane (C_2H_6) is to determine how many valence electrons are available for the molecule. Since C has 4 valence electrons, and each H atoms contributes 1 valence electron, the total number of electrons will be 2*4 + 6*1 = 14 "e"^(-) This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons, either through.
C2H6 lewis structure Etane Hybridization, Molecular Geometry and shape
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

C2h6 Molecule
The Lewis Structure of ethane has two carbons connected to each other, with six carbons surrounding them; three on each carbon atom. There is a single bond b.

Brf3 Polar Or Nonpolar
A step-by-step explanation of how to draw the C2H6 Lewis Dot Structure (Ethane).For the C2H6 structure use the periodic table to find the total number of val.
Lewis Dot Structure Ethane C2h6 Stock Vector (Royalty Free) 2159570631
---- Steps to Write Lewis Structure for compounds like C2H6 ---- 1. Find the total valence electrons for the C2H6 molecule. 2. Put the least electronegative atom in the center. Note:.
33+ C2H6 Lewis Structure Molecular Geometry Gif GM
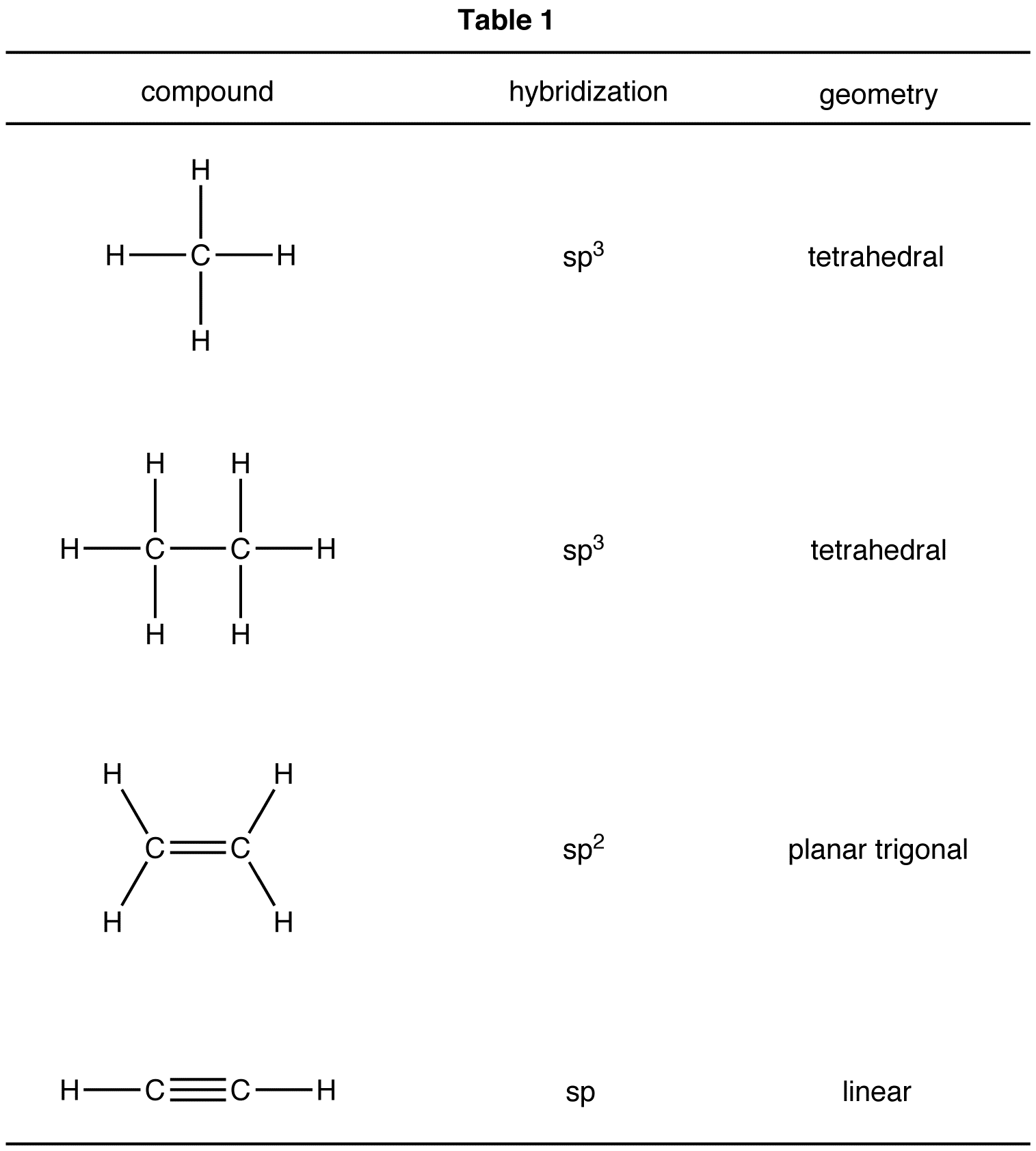
Lewis structure helps with understanding the placement of atoms in the structure along with its valence electrons. The individual atoms with all their valence electrons are shown in this structure to know the bond formation, molecular geometry, and shape of the molecule.

4.6 Introduction to Organic Chemistry The Basics of General, Organic
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for C2H6, ethane. On the periodic table, Carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. So let's multiply that times 2. And then Hydrogen, group 1, one valence electron; we have 6, multiply that by 6, for a total of 14 valence electrons to work with.

Draw the Lewis structures of \ce{C2H6}, \ce{C2H4}, and Quizlet
The Lewis structure of C2H6 (ethane) consists of two carbon atoms and six hydrogen atoms (H)️ at a bond angle of 109.5 degrees.
C2h6 Molecule
Ethane ( US: / ˈɛθeɪn / ETH-ayn, UK: / ˈiː -/ EE-) is a naturally occurring organic chemical compound with chemical formula C 2H 6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining.